How much heat is absorbed when 45.00 g of C(s) reacts in the presence of excess SO2(g) to produce CS2(l) and CO(g) according to the following chemical equation?
5 C(s) + 2 SO2(g) → CS2(l) + 4 CO(g) 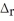
H° = 239.9 kJ
Definitions:
Balance Sheet
A financial statement that provides a snapshot of a company's financial position, showing assets, liabilities, and shareholders' equity at a specific point in time.
Net Income
The total profit of a company after all expenses, including taxes and operational costs, have been subtracted from its total revenue.
Cost of Goods Sold
Direct spending associated with producing the merchandise a company trades, including both materials and manpower costs.
Worksheet
A columnar device used by accountants to aid them in completing the accounting cycle—often just referred to as "spreadsheet." It is not a formal report.
Q9: When 280 mL of 1.50 × 10<sup>-4
Q23: In a Lewis structure,the central atom is
Q36: Give the appropriate values of n and
Q38: Phosphine gas oxidizes spontaneously on exposure to
Q43: A system absorbs 623 J of heat
Q47: How many subshells are there in the
Q63: Calculate the wavelength,in nm,of the first line
Q67: Choose the INCORRECT statement.<br>A)In a Lewis structure,the
Q73: Which of the following has the largest
Q74: Using the VSEPR model,the molecular geometry of