Multiple Choice
When 1.50 mol of CH4(g) reacts with excess Cl2(g) at constant pressure according to the chemical equation shown below,1062 kJ of heat are released.Calculate the value of ΔH for this reaction,as written.
2 CH4(g) + 3 Cl2(g) → 2 CHCl3(l) + 3 H2(g) 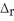 H° = ?
H° = ?
Definitions:
Related Questions
Q15: Which of the following has a molecular
Q15: A liquid has a normal boiling point
Q46: What is the wavelength,in nm,associated with a
Q67: Which of the following has the highest
Q69: Which of the following is a characteristic
Q99: The geometry of sp<sup>2</sup> hybridized orbitals is
Q105: What is the formal charge on B
Q108: Which of the following gases has the
Q113: How many grams of calcium chloride are
Q125: What volume of 6.0 M sulfuric acid