When 2.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere,the reaction shown below occurs and the temperature of the resulting solution rises from 22.00 °C to 40.32 °C.If the specific heat of the solution is 4.18 J g-1°C-1 ,calculate 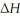 For the reaction,as written.
For the reaction,as written.
Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g)
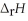 = ?
= ?
Definitions:
Adrenal Glands
Small, triangular glands located on top of the kidneys; they produce hormones that help regulate metabolism, immune system, blood pressure, and stress responses.
Gonads
The primary reproductive organs, ovaries in females and testes in males, producing gametes and sex hormones.
Blood Cells
The cells that circulate in the blood, including red blood cells, white blood cells, and platelets.
Cardiovascular
Pertaining to the heart and blood vessels, and the circulation of blood throughout the body.
Q12: Heat is usually transferred from a cold
Q25: Three types of holes of the cubic
Q30: Oxygen gas at 34.5 °C is compressed
Q44: The ground state electron configuration of Sc
Q53: The following orbitals are placed in the
Q66: In the best Lewis structure for CN<sup>-</sup>,what
Q95: After drawing the Lewis dot structure of
Q110: The condensed electron configuration of sulfur,element 16,is:<br>A)[Ne]3s<sup>2</sup><br>B)[Ar]3s<sup>2</sup><br>C)[Ne]3s<sup>2</sup>3p<sup>4</sup><br>D)[He]2s<sup>4</sup>2p<sup>8</sup><br>E)[He]3s<sup>2</sup>
Q113: When the following equation is completed and
Q120: Flask A has a volume of 0.50