When 50.0 mL of 0.400 mol L-1 Ca(NO3) 2 is added to 50.0 mL of 0.800 mol L-1 NaF,CaF2 precipitates,as shown in the net ionic equation below.The initial temperature of both solutions is 23.0 °C.Assuming that the reaction goes to completion,and that the resulting solution has a mass of 100.00 g and a specific heat of 4.18 J g-1°C-1 ,calculate the final temperature of the solution.
Ca2+(aq) + 2 F-(aq) → CaF2(s) 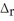 H = -11.5 kJ
H = -11.5 kJ
Definitions:
Osteoporosis
A condition characterized by weakened bones that are more prone to fracture, often occurring with age or hormonal changes.
Metabolic Disease
A broad category of diseases that affect the metabolism, including disorders of carbohydrate metabolism, lipid metabolism, and more, often genetic in nature.
Gout
A form of arthritis caused by excess uric acid in the bloodstream, resulting in painful swelling, typically in the joints of the feet.
Leukemia
A cancer form that hits the blood and bone marrow, resulting in the rampant production of atypical white blood cells.
Q7: Which of the following statements is INCORRECT?<br>A)The
Q52: Use the Balmer equation to determine n
Q57: List in order of increasing number of
Q62: An expansion of gas by a system
Q70: Choose the correct statement about the compound
Q76: All of the terms below are quantum
Q78: The ionization energy is the energy required
Q80: If 356 J of work is done
Q92: A radiation detector exposed to sunlight records
Q115: "One places electrons into orbitals one by