Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) at 35.0 °C to gaseous CCl4 at 76.8 °C (the normal boiling point for CCl4) .The specific heat of CCl4(l) is 0.857 Jg-1°C-1 Its heat of fusion is 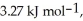 And its heat of vaporization is
And its heat of vaporization is 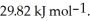
Definitions:
Human Resources Strategy
A plan of action designed to achieve specific organizational goals through the effective management of the workforce, including recruitment, development, and retention of employees.
HR Competencies
Refers to specific knowledge, skills, and abilities required for human resource professionals to effectively contribute to organizational success.
Business Strategy
A plan of action designed to achieve a specific set of objectives and gain a competitive advantage.
Perception Barrier
An obstacle in communication arising from misunderstandings or differences in viewpoints, backgrounds, or personality.
Q7: Nickel has a face-centred cubic structure and
Q18: What is the pH of a 0.30
Q43: The heat of vaporization of water at
Q45: The structure of aspirin is given below.(Note
Q67: Consider the following reaction.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the
Q87: What is the [AsO<sub>4</sub><sup>3-</sup>] for a solution
Q98: The entropy of a system always increases
Q100: Which of the following exhibits ionic bonding?<br>A)C<sub>2</sub>H<sub>6
Q110: Which of the following is a correct
Q119: Which of the following organic substances is