The enthalpy change for converting 10.0 g of ice at -25.0 °C to water at 80.0 °C is ________ kJ.The specific heats of ice,water,and steam are 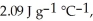
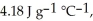 And
And 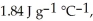 Respectively.For
Respectively.For 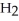 O,
O, 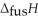 = 6.01 kJ mol-1,and
= 6.01 kJ mol-1,and 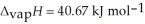 .
.
Definitions:
Peanuts
Edible legumes or seeds enclosed in a hard shell, often consumed as a snack, used in cooking, or processed for oil.
Protein
Organic molecules made up of amino acids that are essential for many processes in living organisms, including catalysis of metabolic reactions and DNA replication.
Generalization
The process of formulating general concepts by abstracting common properties of specific instances, thus allowing for broader applications or interpretations.
Businesses
Organizations engaged in commercial, industrial, or professional activities aimed at generating profits.
Q3: Using the VSEPR model,the molecular geometry of
Q34: What is the weight percent of vitamin
Q43: The heat of vaporization of water at
Q45: Which of the following statements correctly describe
Q67: The Henderson-Hasselbach equation,used to calculate the pH
Q72: For a solute (X)aq,in an ideal aqueous
Q91: A membrane permeable to water but not
Q101: Which of the following is a logical
Q103: Which species in the following reaction acts
Q129: A saturated aqueous solution of calcium hydroxide