In the Haber process,ammonia is synthesized from nitrogen and hydrogen: 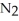 (g) +
(g) + 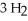 (g) →
(g) → 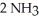 (g)
(g)
ΔrG ° at 298 K for this reaction is -33.3 kJ mol-1.The value of ΔrG at 298 K for a reaction mixture that consists of 1.9 atm 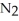 ,1.6 atm
,1.6 atm 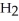 ,and 0.65 atm
,and 0.65 atm 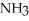 Is ________ kJ mol-1.
Is ________ kJ mol-1.
Definitions:
Air Pressure
The force exerted by air, whether in a specific container or in the atmosphere, typically measured in pounds per square inch (PSI) or bar.
Warning Devices
Equipment or systems designed to alert or notify individuals of potential hazards or dangers.
Ignition Key
A key used to start a vehicle's engine by activating the ignition system.
FMVSS 121
United States Federal Motor Vehicle Safety Standard addressing air brake systems, aiming to ensure safety and reliability of braking in commercial vehicles.
Q17: According to the Arrhenius theory,a neutralization reaction
Q37: The color change range of most acid-base
Q57: What is the [K<sup>+</sup>] of a solution
Q58: Which compound is likely to be the
Q72: What is the pH of a 0.240
Q74: Which of the following pairs of liquids
Q86: What is Δ<sub>r</sub>G°?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="What is Δ<sub>r</sub>G°?
Q90: Which statement regarding VB theory is INCORRECT?<br>A)d
Q91: After drawing the Lewis dot structure for
Q99: A spontaneous process will occur only if