Consider the reaction: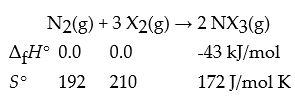
What is ΔrG° for this reaction at 591 K? Is the reaction spontaneous at 591 K?
Definitions:
Net Present Value
A financial measure that calculates the present value of net cash flows (inflows minus outflows) from an investment, discounting future cash flows to the present.
Internal Rate of Return
A metric used in financial analysis to estimate the profitability of potential investments, calculated as the rate that makes the net present value of cash flows equal to zero.
Capital Expenditure Decision Process
The process of making decisions related to the acquisition of capital assets, taking into consideration their costs, benefits, and risks.
Profitability Index
A financial metric that compares the present value of future cash flows from an investment to its initial cost, used to evaluate investment attractiveness.
Q3: What is the ground state electron configuration
Q12: Using the VSEPR model,the molecular geometry of
Q42: Which of the following compounds exhibits only
Q50: A non spontaneous reaction can be made
Q75: Polar bonds are caused by the bonding
Q80: Choose the INCORRECT statement about H<sub>3</sub>O<sup>+</sup>.<br>A)There is
Q97: Which of the following best expresses the
Q98: Which one of the following salts,when dissolved
Q99: How many bond pairs ("bp")and how many
Q121: Assuming no volume change on mixing,what mass