Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) at 35.0 °C to gaseous CCl4 at 76.8 °C (the normal boiling point for CCl4) .The specific heat of CCl4(l) is 0.857 Jg-1°C-1 Its heat of fusion is 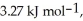 And its heat of vaporization is
And its heat of vaporization is 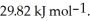
Definitions:
Self-Fulfilling Prophecy
A prediction that ensures, by the behavior it generates, that it will come true.
Counterfactual Thinking
The psychological process of imagining alternative outcomes or scenarios that did not actually happen, often starting with "What if..."
Regret
A feeling of sadness, repentance, or disappointment over something that one has done or failed to do.
False Consensus Effect
The cognitive bias to overestimate how much others share one’s beliefs, attitudes, and behaviors.
Q21: For an exothermic reaction to be non
Q21: Reaction quotient Q will always be equal
Q24: The vapor pressures of pure propyl alcohol
Q29: According to the molecular orbital (MO)theory,when two
Q38: What is the pH of a 0.052
Q56: What is the bond order for the
Q88: A weak acid has K<sub>a</sub> = 4.2
Q88: For the isomerization reaction:<br>Butane ⇌ isobutane<br>K<sub>p</sub> equals
Q98: Equilibrium constant K is constant except when
Q99: The geometry of sp<sup>2</sup> hybridized orbitals is