The enthalpy change for converting 1.00 mol of ice at -50.0 °C to water at 70.0 °C is  The specific heats of ice,water,and steam are
The specific heats of ice,water,and steam are 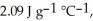
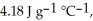 And
And 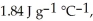 Respectively.For
Respectively.For 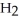 O,
O, 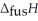 = 6.01 kJ mol-1,and
= 6.01 kJ mol-1,and 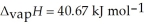 .
.
Definitions:
Pedialyte
An oral electrolyte solution designed to prevent dehydration by replenishing fluids and minerals lost during illness or exercise.
Diarrhea
Frequent, loose, or watery bowel movements, which may be a symptom of infection, digestive disorders, or adverse reaction to food or medication.
Coffee
A popular beverage made from roasted and ground seeds of the Coffea plant, known for its stimulating effect due to caffeine.
Angiotensin-Converting Enzyme
An enzyme that plays a key role in the body's regulation of blood pressure by converting angiotensin I to angiotensin II.
Q1: Write the equilibrium constant expression for the
Q4: For the reaction: CO(g)+ 2 H<sub>2</sub>(g)→ CH<sub>3</sub>OH(g)<br>K<sub>p</sub>
Q9: Hypochlorous acid (HOCl)has an ionization constant of
Q60: Which of the following ionic compounds should
Q94: A triple bond is two sigma bonds
Q103: How much heat would be released by
Q110: Which of the following is a correct
Q116: Which compound is likely to be the
Q122: In the reaction BF<sub>3</sub> + NH<sub>3</sub> ⇌
Q135: What is the pH of an aqueous