The enthalpy change for converting 10.0 g of ice at -25.0 °C to water at 80.0 °C is ________ kJ.The specific heats of ice,water,and steam are 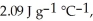
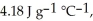 And
And 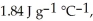 Respectively.For
Respectively.For 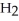 O,
O, 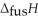 = 6.01 kJ mol-1,and
= 6.01 kJ mol-1,and 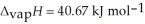 .
.
Definitions:
Bail
A thing of value, such as a money bail bond or any other form of property, that is given to the court to allow a person’s temporary release from jail and to ensure his or her appearance in court.
Offeree
A person or entity to whom an offer is made, which they can either accept or reject.
Offeror
The individual or party that presents a proposal or contract to another party for acceptance.
Reject
To refuse to accept, submit to, believe, or make use of.
Q9: The normal melting and boiling points of
Q9: After drawing the Lewis dot structure of
Q16: For the following chemical equilibrium,K<sub>p</sub> = 4.6
Q19: The ionization constant for ammonia is 1.8
Q39: The electronegativity is 2.1 for H and
Q86: For the reaction: 3 Fe(s)+ 4 H<sub>2</sub>O(g)⇌
Q87: The hybrid orbital set used by the
Q88: Based on the figure above,the boiling point
Q96: What is the molality of a glucose
Q122: Mixing comparable amounts of a strong acid