Calculate the freezing point of a solution of 40.0 g methyl salicylate,C7H6O2,dissolved in 800.g of benzene,C6H6. 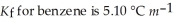 And the freezing point is 5.50 °C for benzene.
And the freezing point is 5.50 °C for benzene.
Definitions:
Distributed
The process of spreading things or people over a wide area or among a large number of recipients.
Enthymeme
A rhetorical form of argument wherein one premise is not explicitly stated, relying on the audience's ability to fill in the gap.
Validity
A property of logical or analytical statements where the conclusion logically follows from the premises.
Unstated Premise
An implicit assumption or belief that is not explicitly expressed but is necessary for an argument's conclusion to hold.
Q3: Given the following bond lengths in pm:<br>N-N,145;N
Q5: Which of the following statements concerning the
Q10: The solubility of cerium iodate,Ce(IO<sub>3</sub>)<sub>3</sub>,molar mass =
Q27: Given the bond enthalpies C =O (707),O
Q41: A solution of LiCl in water has
Q64: How many H<sup>-</sup> ions are around each
Q76: The heat of deposition equals the negative
Q77: 25 mL of 0.10 M aqueous acetic
Q113: Phenol red indicator changes from yellow to
Q123: The vapor pressures of pure hexane and