A solution is prepared by dissolving 7.00 g of glycerin ( C3H8O3) in 201 g of ethanol 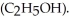 The freezing point of the solution is ________ °C.The freezing point of pure ethanol is
The freezing point of the solution is ________ °C.The freezing point of pure ethanol is 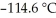 .The molal freezing point depression constant (
.The molal freezing point depression constant ( 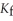 ) for ethanol is
) for ethanol is 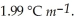 The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
Definitions:
Patent
A government grant that gives an inventor the exclusive right or privilege to make, use, or sell an invention for a limited time period.
Copyright
The exclusive right of an author or other creator to publish, print, or sell an intellectual production for a statutory period of time.
Employment Law
The branch of law that deals with the rights and duties between employers and workers.
Sexual Harassment
In the employment context, the granting of job promotions or other benefits in return for sexual favors (quid pro quo harassment), or language or conduct that is so sexually offensive that it creates a hostile working environment (hostile environment harassment).
Q18: 0.375 g of a monoprotic acid (MW
Q48: What is the pH of a buffer
Q52: Proton acceptor is an abbreviated definition of:<br>A)Br∅nsted-Lowry
Q70: At a certain temperature,nitogen and hydrogen react
Q80: According to Le Chatelier's Principle:<br>A)an increase in
Q95: For the reaction I<sub>2</sub>(s)+ Cl<sub>2</sub>(g)→ 2 ICl(g),Δ<sub>r</sub>H
Q97: What volume in mL of 2.0 M
Q118: Consider the following equation:<br>N<sub>2</sub>O<sub>4</sub>(g)⇌ 2 NO<sub>2</sub>(g),K<sub>c</sub> =
Q119: Which of the following has the largest
Q120: Order the following by increasing entropy.<br>CO(g),COCl<sub>2</sub>(g),CO<sub>2</sub>(g),CaO(s)<br>A)CO<sub>2</sub> <