Calculate the freezing point of a solution containing 5.0 grams of KCl and 550.0 grams of water.The molal freezing point depression constant ( 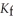 ) for water is
) for water is 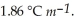
Definitions:
Hospitalized Patients
Individuals who are admitted to a hospital for treatment and observation.
Medicare Part A
A part of the Medicare program in the United States that covers hospital stays, nursing facility care, hospice care, and some home health care services.
Benefit Period
A defined duration during which benefits are paid under the terms of an insurance policy or government program.
Medicare Part B
Medicare Part B is a part of the Medicare program in the United States, covering medical services and supplies, outpatient care, and preventive services.
Q9: What is the free Cu<sup>2+</sup>(aq)concentration if 0.020
Q18: What is the pH of a 0.30
Q29: 3.2 grams of a compound with a
Q54: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="The For
Q62: The solubility product constant for iron(III)hydroxide at
Q73: Unhybridized p orbitals must be present for
Q75: Which of the following substances present in
Q89: The following compounds are available as 0.10
Q116: Arrange in order by decreasing boiling point:<br>Cl<sub>2</sub>,I<sub>2</sub>,HI<br>A)I<sub>2</sub>,Cl<sub>2</sub>,HI<br>B)Cl<sub>2</sub>,I<sub>2</sub>,HI<br>C)Cl<sub>2</sub>,HI,I<sub>2
Q119: The enthalpy of vaporization at 298 K