A solution is prepared by dissolving 7.00 g of glycerin ( C3H8O3) in 201 g of ethanol 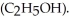 The freezing point of the solution is ________ °C.The freezing point of pure ethanol is
The freezing point of the solution is ________ °C.The freezing point of pure ethanol is 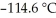 .The molal freezing point depression constant (
.The molal freezing point depression constant ( 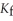 ) for ethanol is
) for ethanol is 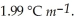 The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1,respectively.
Definitions:
Night Terrors
Episodes of intense fear that occur during deep (non-REM) sleep, typically in children, characterized by screaming, sweating, confusion, and a heightened heart rate, without memory of the event upon waking.
Restorative Function
A theory proposing that sleep serves to repair and rejuvenate the body and mind, restoring physiological and psychological functions.
Consciousness
The state of being aware of and able to think about one's own existence, sensations, thoughts, surroundings, and experiences.
Non-REM
Describes a type of sleep that is divided into stages, excluding the REM (Rapid Eye Movement) phase, characterized by deeper physical and mental rest.
Q4: The heat of deposition equals the negative
Q23: Choose the INCORRECT statement.<br>A)The third law of
Q34: The solubility of CaF<sub>2</sub> is 0.00021 mole
Q35: Predict the molar solubility of the following
Q55: Cyclohexane,C<sub>6</sub>H<sub>12</sub>,undergoes a molecular rearrangement in the presence
Q71: Phosphorus and chlorine gases combine to produce
Q83: Choose the INCORRECT statement.The term pH:<br>A)refers to
Q99: The geometry of sp<sup>2</sup> hybridized orbitals is
Q101: What would be the bond order of
Q111: What is the pH of an aqueous