Consider the equilibrium system: N2O4(g) ⇌ 2 NO2(g) for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assuming that the total pressure inside the container is 1.00 atm at equilibrium and that initially only N2O4 was present inside the container,compute 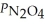
At equilibrium.
Definitions:
Uncontrollable Aggression
An intense and often violent form of behavior where an individual is unable to regulate their angry impulses.
Social Psychology
The scholarship surrounding how individuals’ minds, emotions, and actions are directed by the presence of others, be it actual, fictional, or intimated.
Suicide
The act of intentionally causing one's own death, often as a response to distress, mental health conditions, or feelings of hopelessness.
Instinct Theories
Theories suggesting that behavior is driven by innate, biologically determined instincts, which are automatic and unlearned responses to stimuli.
Q15: The vapor pressures of pure propyl alcohol
Q26: What is the pH of a 0.570
Q47: What molar concentration of silver ion could
Q54: The phenomenon of supercooling refers to the
Q69: Gold crystallizes in a face-centred cubic structure.What
Q92: Colligative properties are similar in that they
Q96: When equal volumes of the indicated aqueous
Q105: For NaC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>,predict whether the aqueous solution is
Q106: When dissolved in water,which compound is generally
Q114: For the reaction: Mg(s)+ AgNO<sub>3</sub>(aq)→ Ag(s)+ Mg(NO<sub>3</sub>)<sub>2</sub>(aq)<br>Ag<sup>+</sup>(aq)+