Consider the equilibrium system: N2O4(g) ⇌ 2 NO2(g) for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assuming that the total pressure inside the container is 10 atm at equilibrium and that initially only N2O4 was present inside the container,compute 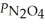
At equilibrium.
Definitions:
Common Bond
A shared connection or interest that unites members of a group or community.
Entitativity
The degree to which a group is perceived as being a coherent, unified entity.
Ingroup Bias
A tendency to favor one's own group over other groups.
Ingroup
A social group to which a person psychologically identifies as being a member.
Q2: HNO<sub>3</sub> is a strong acid.
Q10: NaCl crystallizes in a cubic unit cell
Q18: How many coulombs would be needed to
Q50: A sweetened cup of coffee is an
Q60: The HCOO<sup>-</sup> ion can be described by
Q65: A solution that can dissolve no more
Q69: Choose the INCORRECT statement.<br>A)Colloidal particles are large
Q78: Large value for equilibrium constant K means
Q85: A KCl solution is prepared by dissolving
Q117: For CO(g)+ H<sub>2</sub>(g)→ H<sub>2</sub>CO(g),Δ<sub>r</sub>H° = -5.36 kJ/mol,and