The 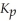 For the reaction below is 1.49 × 108 at 100.0 °C:
For the reaction below is 1.49 × 108 at 100.0 °C:
CO(g) +Cl2(g) →COCl(g)
In an equilibrium mixture of the three gases, PCO=PCl2= 2.22 × 10-4 bar.The partial pressure of the product,phosgene ( COCl2) ,is ________ bar.
Definitions:
Service-sponsored Franchise Systems
A franchising approach where the franchisor provides a comprehensive service package to the franchisee, including training, branding, and ongoing support, typically seen in hospitality and retail industries.
Government-sponsored
Programs, initiatives, or projects that are funded or promoted by a governmental entity.
Customer-oriented
An approach or strategy that focuses primarily on meeting the needs and expectations of customers.
Retailer-sponsored Cooperatives
Organizations owned and operated by a group of retailers aimed at improving purchasing power and marketing capabilities through collective efforts.
Q15: Consider the reaction: AB(g)→ A(g)+ B(g)<br>(K<sub>eq</sub> =
Q53: What is the pH of a solution
Q64: A solution prepared by dissolving 4.00 g
Q67: Which of the following forms an ionic
Q76: Commercial perchloric acid is 70.0% by mass,HClO<sub>4</sub>(aq),and
Q78: What molality of pentane is obtained by
Q79: What is the pH of an aqueous
Q94: Write the solubility product constant expression for
Q122: A solid solution of zinc in copper
Q131: What is the pH of a 0.475