At 900.0 K,the equilibrium constant (Kp) for the following reaction is 0.345: 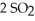 +
+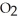 (g) →
(g) → 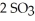 (g)
(g)
At equilibrium,the partial pressure of 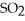 Is 35.0 bar and that of
Is 35.0 bar and that of 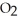 Is 15.9 bar.The partial pressure of
Is 15.9 bar.The partial pressure of 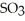 Is ________ bar.
Is ________ bar.
Definitions:
Federal Level
Pertaining to the national government or aspects of governance that involve the entire country as opposed to local or state levels.
Aid Program
Initiatives designed to provide financial or material assistance to individuals, groups, or countries in need, often administered by governments or charitable organizations.
Unemployment Benefits
Payments made by the government to unemployed individuals who meet certain eligibility requirements.
Department of Agriculture
A government department responsible for developing and executing federal policies on farming, agriculture, forestry, and food, aiming to meet the needs of farmers and ensure food safety.
Q1: What is the pH of a 0.250
Q9: Hypochlorous acid (HOCl)has an ionization constant of
Q15: The vapor pressures of pure propyl alcohol
Q20: Using the VSEPR model,the electron-group geometry of
Q27: List the following acids in order of
Q30: The orbital hybridization on the carbon atoms
Q79: For a reaction,the reaction quotient,Q<sub>c</sub> > K<sub>c</sub>
Q80: Choose the Br∅nsted-Lowry acids and bases in
Q101: The value of Δ<sub>f</sub>G at 100.0 °C
Q102: Determine the pH of 263 ml of