Dinitrogen tetroxide partially decomposes according to the following equilibrium: 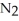
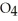 (g) ⇌
(g) ⇌ 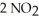 (g)
(g)
A 1.000 L flask is charged with 3.00 × 10-2 mol of 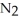
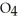 .At equilibrium,2.36 × 10-2 mol of
.At equilibrium,2.36 × 10-2 mol of 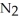
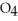 Remains.
Remains. 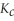 For this reaction is:
For this reaction is:
Definitions:
Diversification
An investment strategy that minimizes risk by allocating assets among various financial instruments, industries, or other categories.
Market Risk
Risk that affects all companies in the stock market.
Portfolio
A collection of financial investments like stocks, bonds, commodities, cash, and cash equivalents, including closed-end funds and exchange traded funds (ETFs).
Return on Stocks
The earnings generated from investing in stocks, typically measured in terms of dividend payments and capital gains.
Q10: NaCl crystallizes in a cubic unit cell
Q39: There are no types of crystalline solids
Q49: Consider the following reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the
Q61: A 0.0925 g sample of a monoprotic
Q92: The standard Gibbs energy change for the
Q97: For the reaction: N<sub>2</sub>(g)+ 2 O<sub>2</sub>(g)⇌ 2
Q101: A constant current of 10.0 A is
Q101: The solubility of ammonium permanganate is 7.90
Q106: What is Δ<sub>r</sub>G° at 25 °C?<br>CO<sub>2</sub>(g)→ CO<sub>2</sub>(aq)Δ<sub>r</sub>H°
Q108: In a reaction at equilibrium involving only