What is the hydronium ion concentration and the pH for an aqueous solution of NH3 that has a hydroxide ion concentration of 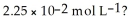
Definitions:
Stockholders' Equity
Represents the equity stake currently held on a company's balance sheet by its shareholders, often calculated as total assets minus total liabilities.
Paid-In Capital
refers to the funds received from shareholders in exchange for shares of stock, representing investment directly into the company.
Organization Costs
Expenses incurred during the establishment of a corporation or entity, including legal fees, registration fees, and promotional expenses.
Intangible Asset
An asset that lacks physical substance, representing value due to legal or competitive rights (e.g., patents, trademarks).
Q11: E°<sub>cell</sub> is the standard cell potential.
Q12: Which of the following are commonly used
Q44: In a reaction at equilibrium involving only
Q63: For the reaction: CH<sub>4</sub>(g)+ 2 H<sub>2</sub>O(g)⇌ CO<sub>2</sub>(g)+
Q86: If the rate of a specific chemical
Q88: Calculate E<sub>cell </sub>at 25 °C for the
Q98: A solution of nonvolatile solute has an
Q99: What volume in mL of 0.05 M
Q115: What is Δ<sub>r</sub>G° at 25 °C?<br>N<sub>2</sub>O<sub>4</sub>(g)→ 2
Q124: For the reaction: Mg(s)+ AgNO<sub>3</sub>(aq)→ Ag(s)+ Mg(NO<sub>3</sub>)<sub>2</sub>(aq)<br>Ag<sup>+</sup>(aq)+