What is the hydronium ion concentration of a 0.150 mol L-1 aqueous hypochlorous acid solution with 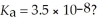
The equation for the dissociation of hypochlorous acid is below: 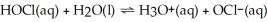
Definitions:
Runs Out
A situation where a supply or quantity of something is completely used up or exhausted.
Next Century
A future period of 100 years, used to forecast or plan long-term visions or developments.
Economic Viability
The ability of an entity or activity to sustain itself financially over the long term.
Alternatives
Different options or choices available in a given situation, often considered when making decisions.
Q11: What is the [CH<sub>3</sub>COO<sup>-</sup>]/[CH<sub>3</sub>COOH] ratio necessary to
Q32: In the equilibrium system described by: <br><img
Q39: For the following titration,determine whether the solution
Q57: Determine E°<sub>cell</sub> at 25 °C for the
Q58: 0.272 g of a monoprotic acid (MW
Q60: A swimming pool was sufficiently alkaline so
Q85: In a qualitative cation analysis,the unknown ion
Q89: What is the shorthand notation that represents
Q105: Consider the reaction: AB(g)? A(g)+ B(g)<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg"
Q110: What is the [Cl<sup>-</sup>] of a solution