Calculate the pH of a 1.60 mol L-1 aqueous KBrO solution.Ka for hypobromous acid,HBrO,is 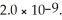
Definitions:
Abhijit Banerjee
An Indian-American economist who, along with Esther Duflo and Michael Kremer, won the Nobel Prize in Economic Sciences in 2019 for their work in development economics.
Extremely Poor
Individuals or households whose income level is significantly below the poverty line, making it difficult to meet basic needs.
Opportunity Cost
The economic consequence of bypassing the immediate superior alternative in decision-making.
Labor Force
The total number of people employed or actively seeking employment within an economy.
Q1: What is the pH of a 0.250
Q1: What mass of sodium acetate should be
Q6: Consider the equilibrium system: N<sub>2</sub>O<sub>4</sub>(g)⇌ 2 NO<sub>2</sub>(g)for
Q33: In the reaction BF<sub>3</sub> + NH<sub>3</sub> →
Q60: 1.80 grams of an impure mixture containing
Q62: Choose the INCORRECT statement.<br>A)A concentration cell consists
Q84: Calculate the hydronium ion concentration in an
Q90: The reaction 2 H<sub>2</sub> + NO →
Q101: A constant current of 10.0 A is
Q121: How many grams of KBr are required