Determine the ammonia concentration of an aqueous solution that has a pH of 11.00.The equation for the dissociation of NH3 (Kb = 1.8 × 10-5) is below: 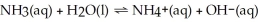
Definitions:
Tautology
An expression or phrase that redundantly states the same concept in different words, effectively redundant in its logical or semantic content.
Grammatical Construction
The arrangement and relationship of words, phrases, and clauses in a sentence to convey meaning effectively.
Necessarily True
A statement that cannot be false under any circumstance.
Q4: The equilibrium constant,K<sub>p</sub>,equals 3.40 for the isomerization
Q53: At 20 °C,a 0.376 mol L<sup>-1</sup> aqueous
Q59: An azeotropic mixture is a:<br>A)mixture of two
Q90: For the following voltaic cell,determine the [Cl<sup>-</sup>]
Q99: Which of the following are Lewis bases?<br>I.BCl<sub>3
Q105: Which of the following is an example
Q108: For the first-order reaction,2 N<sub>2</sub>O(g)→ 2 N<sub>2</sub>(g)+
Q119: A 25.0 mL sample of 0.150 mol
Q121: How many grams of KBr are required
Q123: What is the buffer range (for an