Determine the ammonia concentration of an aqueous solution that has a pH of 11.00.The equation for the dissociation of NH3 (Kb = 1.8 × 10-5) is below: 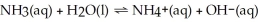
Definitions:
Negligence Action
A legal claim brought against someone who fails to act with the level of care that someone of ordinary prudence would have exercised under the same circumstances, resulting in harm or damage.
Building Owner
An individual or entity that holds title to a building or property.
Economic Loss
A financial loss suffered by a person or organization, often due to damage or negligence.
General Duty
An obligation imposed on individuals or organizations to adhere to a standard of reasonable care while performing any acts that could foreseeably harm others.
Q16: What is the pH of a 0.530
Q17: The isomerization of methylisonitrile to acetonitrile <br><img
Q42: What is the approximate pH at the
Q46: An aqueous solution has [HC<sub>7</sub>H<sub>5</sub>O<sub>2</sub>] = 0.100
Q50: Determine the pH of the following aqueous
Q72: If the vapor pressure of water in
Q74: What will the pH at the neutralization
Q82: The pH of an aqueous solution at
Q94: Determine the pH of the following aqueous
Q102: Calculate the entropy change for methanol at