Multiple Choice
Calculate the pH of a 0.60 mol L-1 aqueous H2SO3 solution that has the stepwise dissociation constants Ka1 = 1.5 × 10-2 and 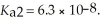
Definitions:
Related Questions
Q1: Ethanol ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Ethanol (
Q2: At a given temperature the vapour pressures
Q6: How long must a constant current of
Q15: Consider the reaction: AB(g)→ A(g)+ B(g)<br>(K<sub>eq</sub> =
Q59: What is the pH of a 0.40
Q98: A solution of nonvolatile solute has an
Q106: A mixture,containing 0.0750 M HCl(g)and 0.0330 M
Q113: How many grams of chromium metal are
Q114: In a solution prepared by mixing equal
Q116: What will happen to the equilibrium in