Calculate the pH of an aqueous solution that is 0.210 mol L-1 in nitrous acid ( 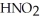 ) and 0.290 mol L-1 in potassium nitrite (
) and 0.290 mol L-1 in potassium nitrite ( 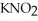 ) .The acid dissociation constant of nitrous acid is 4.50 ×
) .The acid dissociation constant of nitrous acid is 4.50 × 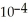 .
.
Definitions:
Educators
Educators are individuals who teach, instruct, or facilitate learning, typically within a formal educational setting such as a school or university.
Cultural Stereotypes
Oversimplified and generalized beliefs or opinions about characteristics, attributes, and behaviors of members of a particular culture.
Strategies
Plans or methods designed for achieving specific goals or outcomes.
Authoritative Parenting
A parenting style characterized by high responsiveness and high demands, promoting independence while setting clear standards.
Q28: Consider the exothermic reaction:<br>4 HCl(aq)+ MnO<sub>2</sub>(s)⇌ Cl<sub>2</sub>(g)+
Q28: What is the mole fraction of oxygen
Q39: For the reaction: 3 Fe(s)+ 4 H<sub>2</sub>O(g)⇌
Q50: An aqueous solution of boric acid can
Q68: The rate of disappearance of HBr in
Q86: The solubility of a salt MX<sub>2</sub> with
Q91: A solution of Al(NO<sub>3</sub>)<sub>3</sub>(aq)is electrolyzed by passing
Q99: What volume in mL of 0.05 M
Q114: Choose the INCORRECT statement about the inert-pair
Q126: What is the E<sub>cell</sub> for the following