What is the hydronium ion concentration in an aqueous solution prepared by mixing 50.00 mL of 0.10 mol L-1 HCN with 50.00 mL of 0.010 mol L-1 NaCN? Assume that the volumes of the solutions are additive and that Ka = 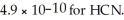
Definitions:
Flexible Work
Employment arrangements that allow for variations in work schedule, location, and hours, catering to the needs and preferences of employees.
Telecommuting
The practice of working from a remote location, typically one's home, using the internet and telecommunications technology.
Job Performance
The evaluation of how effectively an employee carries out their assigned tasks and duties.
Perceptions of Autonomy
The degree to which individuals feel they have control or freedom over their work and decisions within an organization.
Q3: 25 mL of 0.10 M aqueous acetic
Q19: Which of the following statements correctly describe
Q21: Choose the INCORRECT statement.<br>A)Group 2 (2A)compounds are
Q28: For the reaction: C<sub>2</sub>H<sub>4</sub>Br<sub>2</sub> + 3 KI
Q61: A 0.0925 g sample of a monoprotic
Q83: A magnesium sulfate heptahydrate solution,which is 18.00%
Q86: If the rate of a specific chemical
Q92: How many mL of 0.200 M aqueous
Q94: Write the solubility product constant expression for
Q125: The freezing point of a solution made