An aqueous solution is prepared by dissolving 0.23 mol of nitrous acid and 0.27 mol of sodium nitrite in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly.The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution.The 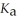
Of nitrous acid is 1.36 × 10-3.
Definitions:
Exclusiveness
In the context of relationships, it refers to being romantically involved with one person only, not maintaining or seeking other sexual or romantic relationships.
Hermaphrodite Couples
A term, often considered outdated and inaccurate, historically used to describe couples where one or both partners have intersex traits.
Gay Couples
Romantic partnerships between two individuals of the same sex.
Q1: At 20 °C,a 2.32 mol L<sup>-1</sup> aqueous
Q15: What is the rate law for the
Q27: For the decomposition of SO<sub>3</sub>(g),K<sub>c</sub> = [SO<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>]/[SO<sub>3</sub>]<sup>2</sup>,at
Q37: Liquid Q is a polar solvent and
Q42: The first-order reaction,2 N<sub>2</sub>O(g)→ 2 N<sub>2</sub>(g)+ O<sub>2</sub>(g),has
Q44: Two solutions contain the same amount of
Q45: What is the pH of a solution
Q59: An aqueous solution has [HC<sub>7</sub>H<sub>5</sub>O<sub>2</sub>] = 0.100
Q94: For the reaction 2 NO<sub>2</sub>(g)⇌ N<sub>2</sub>O<sub>4</sub>(g)K<sub>p</sub> equals
Q126: Which of the following is the strongest