Calculate the pH of an aqueous solution that is 0.210 mol L-1 in nitrous acid ( 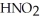 ) and 0.290 mol L-1 in potassium nitrite (
) and 0.290 mol L-1 in potassium nitrite ( 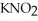 ) .The acid dissociation constant of nitrous acid is 4.50 ×
) .The acid dissociation constant of nitrous acid is 4.50 × 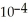 .
.
Definitions:
Yield
The amount of product produced per unit area or per process.
Wheat Varieties
Different types or classifications of wheat, each with unique characteristics, growth patterns, and adaptation to climates.
Randomized Block Design
A statistical experiment design that aims to reduce the effects of confounding variables by dividing subjects into blocks before randomly assigning treatments.
Soil Type
A classification of soil based on its physical and chemical properties, such as texture, structure, and nutrient content.
Q7: Which of the following metals is refined
Q35: Predict the molar solubility of the following
Q46: What is ΔG° at 25 °C for
Q79: What is the shorthand notation that represents
Q81: What volume of oxygen gas,measured at STP,would
Q95: If some NH<sub>4</sub>Cl is added to an
Q98: Which one of the following salts,when dissolved
Q100: Strontium is a softer metal than calcium.
Q105: For NaC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>,predict whether the aqueous solution is
Q135: What is the pH of an aqueous