Calculate the percent ionization of nitrous acid in an aqueous solution that is 0.266 mol L-1 in nitrous acid 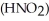 And 0.112 mol L-1 in potassium nitrite (
And 0.112 mol L-1 in potassium nitrite ( 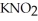 ) .The acid dissociation constant of nitrous acid is
) .The acid dissociation constant of nitrous acid is 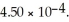
Definitions:
Lactic Acid
A substance produced in the muscles during strenuous exercise, often associated with muscle fatigue.
Contract
A voluntary agreement between two parties in which specific promises are made.
ATP
Adenosine triphosphate, an organic compound that provides energy to drive many processes in living cells, including muscle contraction and chemical synthesis.
Energy Production
The process of generating electricity or other forms of energy through various means such as fossil fuels, nuclear power, or renewable resources.
Q2: What is the pH of a buffer
Q41: A primary battery is recharged by passing
Q60: If the half-life of a reaction depends
Q68: We wish to lower the freezing point
Q79: What is the free Ag<sup>+</sup>(aq)concentration of 0.020
Q86: A 250.0 ml sample of gaseous hydrogen
Q91: A membrane permeable to water but not
Q110: For CO<sub>2</sub>(g)+ H<sub>2</sub>(g)⇌ CO(g)+ H<sub>2</sub>O(g),K<sub>c</sub> = [CO][H<sub>2</sub>]/[CO<sub>2</sub>][H<sub>2</sub>],if
Q114: For the reaction: Mg(s)+ AgNO<sub>3</sub>(aq)→ Ag(s)+ Mg(NO<sub>3</sub>)<sub>2</sub>(aq)<br>Ag<sup>+</sup>(aq)+
Q132: Molality is independent of temperature,molarity is dependent