What is the pH of a buffer solution that is 0.255 mol L-1 in hypochlorous acid (HClO) and 0.333 mol L-1 in sodium hypochlorite (NaClO) ?
The 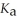 Of hypochlorous acid is 3.8 ×
Of hypochlorous acid is 3.8 × 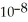 .
.
Definitions:
Tradeable Property Rights
Legal rights that allow owners to sell, lease, or otherwise transfer their property or its use to others.
Monopoly
An economic scenario where only one seller exists who offers a distinctive product to the marketplace.
Price Discrimination
A pricing strategy where different prices are charged for the same product or service in different markets or to different consumers.
Elastic Demand
A situation where the quantity demanded of a product is highly responsive to changes in its price.
Q8: In a qualitative cation analysis,the unknown ion
Q14: For a galvanic cell that uses the
Q20: The stronger the forces between solute and
Q35: List the following acids in order of
Q62: How many moles of ethylene glycol must
Q69: For the reaction: 2 N<sub>2</sub>O<sub>5</sub>(g)→ 4 NO<sub>2</sub>(g)+
Q103: What is Keq at 25 °C of
Q104: If the voltage of an electrochemical cell
Q111: For the reaction below<br>CO(g)+ 2 H<sub>2</sub>(g)⇌ CH<sub>3</sub>OH(g)<br>The
Q128: What is the hydronium ion concentration and