A concentrated buffer of pH 8.0 is added to an equal volume of an aqueous solution that is 0.080 M in each of the ions Zn2+,Ni2+,and Mn2+.The expected precipitate would consist of ________.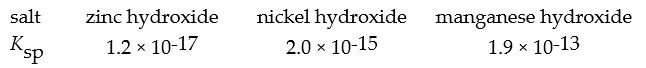
Definitions:
Intangible Benefit
Non-quantifiable advantages that cannot be measured in monetary terms, such as brand recognition or employee satisfaction.
Discount Rate
The interest rate used to determine the present value of future cash flows.
Above-Ground Pipelines
Pipelines that are constructed and operate on the surface of the ground as opposed to being buried underground.
Annual Cash Inflow
The total amount of money received by a company from its various activities, including sales, investments, and financing, within a year.
Q7: Which of the following metals is refined
Q28: Consider the exothermic reaction:<br>4 HCl(aq)+ MnO<sub>2</sub>(s)⇌ Cl<sub>2</sub>(g)+
Q43: Calculate the concentration of bicarbonate ion,HCO<sub>3</sub><sup>-</sup>,in a
Q43: In a galvanic cell,oxidation occurs at the:<br>A)anode<br>B)cathode<br>C)salt
Q51: What is the value for K<sub>c</sub> if
Q61: Define "hydrogenation reaction."<br>A)Hydrogen is produced chemically.<br>B)Double bonds
Q70: List the following acids in order of
Q75: Which of the following substances present in
Q85: The rate constant,k,for a first-order reaction is
Q86: Which of the following metals has been