What is the molar solubility of Mg(OH) 2(s) in a basic aqueous solution with a pH of 12.50? Ksp for Mg(OH) 2(s) is 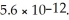
Definitions:
Opportunity Cost
The trade-off of not seizing the next most favorable opportunity when faced with a decision.
Economics Club
A group or organization often found in academic institutions, where members discuss economic theories, current events, and sometimes engage in related projects or competitions.
Accounting Club
An organization or group often found in academic settings that focuses on the study and discussion of accounting practices, career development, and networking for accounting students and professionals.
Marginal Analysis
An examination of the benefits and costs of an additional unit of production or activity to make better decisions.
Q1: How many seconds are required to produce
Q6: Activation energy is:<br>I.the minimum kinetic energy required
Q23: Fe(OH)<sub>3</sub> is most soluble in which aqueous
Q39: For the following titration,determine whether the solution
Q45: Determine E<sub>cell</sub> at 25 °C for the
Q47: Choose the strongest acid.<br>A)HF<br>B)H<sub>2</sub>CO<sub>3 </sub><br>C)HCN<br>D)HC<sub>2</sub>H<sub>3</sub>O<sub>2 </sub><br>E)HClO<sub>4 </sub>
Q53: What is the pH of a solution
Q59: Choose the INCORRECT statement.<br>A)Potassium chloride is used
Q64: For a reaction that follows the general
Q104: What is the pH of a 0.375