What is the molar solubility of AgCl in 0.40 mol L-1 NH3(aq) ? Ksp for AgCl(s) is 1.8 × 10-10 and Kf for Ag(NH3) 2+ is 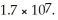
Definitions:
Average Total Cost
The total cost of production divided by the quantity of output produced, representing the average cost per unit of output.
Homogeneous Products
Goods that are identical in quality and cannot be distinguished from one another by consumers.
Free Entry
Free entry is a market condition where businesses can enter and exit the market without facing significant barriers or costs.
Competitive Price-Taker
A firm or individual in a market structure that has no control over the market price and must accept the prevailing market price for its product or service.
Q8: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q13: In a second order reaction:<br>I.the sum of
Q16: What is the pH of a 0.530
Q73: The first ionization step is approximately 100%
Q73: Which of the following exists in more
Q76: Ion pairs as a limitation to K<sub>sp</sub>
Q84: In a qualitative cation analysis,the unknown ion
Q103: Strontium hydroxide is more soluble in water
Q108: In a reaction at equilibrium involving only
Q115: Define rate law.<br>A)A theoretical equation that describes