Multiple Choice
What is the molar solubility of Mg(OH) 2(s) in a basic aqueous solution with a pH of 12.50? Ksp for Mg(OH) 2(s) is 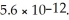
Understand the stages involved in brand ambidexterity and brand transformation.
Identify strategies and outcomes for maintaining authenticity through cultural storytelling and cultural branding models.
Understand the concept of design disruption in branding.
Identify successful strategies to respond to backlash when innovating a brand.
Definitions:
Related Questions
Q11: E°<sub>cell</sub> is the standard cell potential.
Q23: Which of the following keep the equilibrium
Q28: For the reaction: C<sub>2</sub>H<sub>4</sub>Br<sub>2</sub> + 3 KI
Q35: Predict the molar solubility of the following
Q66: The decomposition of ammonia is 2 NH<sub>3</sub>(g)→
Q67: In the stepwise preparation of pure calcium
Q78: Choose the INCORRECT statement.<br>A)The production of limestone
Q85: An aqueous solution at 25.0 °C contains
Q108: Choose the INCORRECT statement.<br>A)An electrode is often
Q121: The K<sub>b</sub> value for methylamine is 4.2