A voltaic cell is constructed with two 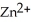 -Zn electrodes,where the half-reaction is
-Zn electrodes,where the half-reaction is 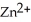 (aq) +
(aq) + 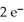 → Zn(s) E° = -0.763 V
→ Zn(s) E° = -0.763 V
The concentrations of zinc ion in the two compartments are 5.50 mol L-1 and 1.11 × 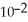
Mol L-1,respectively.The cell emf is ________ V.
Definitions:
Eye Irrigations
The process of flushing or washing out the eyes with a fluid to remove foreign bodies or chemicals.
Lactated Ringer's
A solution used in intravenous therapy for fluid and electrolyte replenishment, containing sodium, chloride, potassium, calcium, and lactate.
Chemical Splash Injury
An injury caused by the exposure of skin or eyes to hazardous chemicals in liquid form, often requiring immediate medical attention.
Irrigation Fluid
A sterile solution used to cleanse or flush a body part, typically during medical procedures to remove debris or bacteria.
Q3: The overall formation constant can be calculated
Q37: Lithium differs from other members of the
Q38: Write the solubility product constant expression for
Q70: List the following acids in order of
Q73: The combustion of ethylene proceeds by the
Q73: Write the equation for the initial treatment
Q78: Data for the reaction A + B
Q98: Choose the correct shape,weak or strong field,and
Q101: For the reaction: 2 N<sub>2</sub>O<sub>5</sub>(g)→ 4 NO<sub>2</sub>(g)+
Q102: What is the rate constant at 305