A voltaic cell is constructed with two 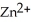 -Zn electrodes,where the half-reaction is
-Zn electrodes,where the half-reaction is 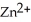 (aq) +
(aq) + 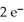 → Zn(s) E° = -0.763 V
→ Zn(s) E° = -0.763 V
The concentrations of zinc ion in the two compartments are 5.50 mol L-1 and 1.11 × 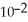
Mol L-1,respectively.The cell emf is ________ V.
Definitions:
Free Trade
International commerce that operates naturally without the interference of tariffs, quotas, or restrictions.
Domestic Consumption
Domestic Consumption is the total amount of goods and services consumed within a country, excluding those used for exports.
Domestic Price
The price of goods or services within a country's domestic market, as opposed to international or export prices.
Free Trade
Worldwide trade unencumbered by tariffs, quotas, or any form of regulatory constraints.
Q12: Which of the following are commonly used
Q15: Determine the [F<sup>-</sup>] of the following aqueous
Q15: What is the pH of a 0.175
Q31: What mass of manganese dioxide,from pyrolusite ore,is
Q36: The solubility product constant of M<sub>2</sub>CrO<sub>4</sub>(s)is 7.11
Q41: A pH 4.88 buffer was prepared by
Q60: Iodine is only very slightly soluble in
Q90: What is the pH of an aqueous
Q104: If the voltage of an electrochemical cell
Q121: A solution of AuCl<sub>3</sub>(aq)is electrolyzed by passing