The following reaction represents the self-ionization of ammonia (NH3).
NH3(aq)+ NH3(aq) 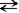 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In this reaction, the conjugate base of ammonia is the _______________________ ion.
Definitions:
Egalitarianism
A political philosophy advocating for equal rights and opportunities for all individuals.
Political Orientation
An individual's or group's predisposition towards particular political beliefs, ideologies, or affiliations.
Schooling
The process of receiving education in an institutional setting, like a school, where academic skills and knowledge are systematically taught.
Conservative Tide
Refers to a period in political history characterized by a shift towards conservative values and policies, often marked by reduced government intervention in the economy and a focus on traditional social norms.
Q3: Lactic acid fermentation supplies less energy than
Q16: One would expect a compound with the
Q17: Structure C is classified as a(n) _.
Q29: Which of the following explains the fact
Q32: Draw the zwitterion ion form of phenylalanine.
Q34: Consider the process illustrated in the image
Q37: Which of the following is probably soluble
Q55: Structure _would produce a single product on
Q58: The monosaccharide shown below would be classified
Q69: Compare the following two metric rulers. Fill