The following reaction represents the self-ionization of ammonia (NH3).
NH3(aq)+ NH3(aq) 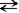 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In the reverse reaction, __________________________ion functions as an acid.
Definitions:
Proximate Cause
The primary cause of an injury in a legal sense, which is directly responsible for the event in question and without which the event would not have occurred.
Injured Ankle
A common physical injury involving harm or damage to the ankle joint or surrounding tissues, possibly impairing mobility.
Automobile Accident
An incident involving one or more vehicles where damage or injury occurs, typically resulting from a collision.
Taxi
A vehicle licensed to transport passengers in return for payment of a fare.
Q1: Structure A is classified as a(n) _.
Q6: The heat of reaction would be represented
Q20: One Hawaiian sweet roll contains:<br>2.5 g fat<br>18
Q20: What is the major product produced upon
Q35: Which of the following statements is generally
Q37: Enter the number (0, 1 ,2, 3,
Q52: Enter the chemical symbol of the element
Q62: The tin compound used to strengthen teeth
Q70: What compound should be used to produce
Q78: The pK<sub>a</sub> of lactic acid is 3.86,