The following reaction represents the self-ionization of ammonia (NH3).
NH3(aq)+ NH3(aq) 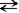 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In this reaction, the conjugate base of ammonia is the _______________________ ion.
Definitions:
Marginal Products
The additional output obtained by employing one more unit of a certain input while keeping other inputs constant.
Separating Equilibrium
A concept in game theory where different types of participants (players) choose different strategies, revealing their type in the process.
Pooling Equilibrium
An outcome where market participants with different characteristics choose indistinguishable actions, making it impossible to differentiate between them based on their choices.
Microeconomics
The branch of economics that studies individual agents and markets, their interactions, and the outcomes of these interactions.
Q13: The order reads 'haloperidol (generic name) 1
Q14: Per gram _ produces the largest amount
Q31: Consider the following reaction. <br>NaOH(aq) + H<sub>2</sub>SO<sub>4</sub>(aq)
Q37: Which of the following contains a sulfur
Q40: The portion of the molecule in the
Q42: When a nickel-cadmium battery is recharged, the
Q47: An enzyme found in the blood would
Q51: The structure for dipropyl ether is: <img
Q58: The line structure for <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt="The
Q78: Draw the Lewis structure for the following