Nickel has a lower atomic mass than cobalt, even though it has a higher atomic number.One possible explanation is that one of the average atomic masses was miscalculated.In the case of cobalt, there is only one isotope: 100% 59Co at a mass of 58.9332 amu.For nickel, however, there are five isotopes as given in the table. 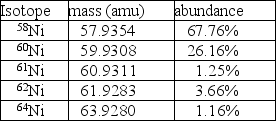 A.Using the data in the table, calculate the average atomic mass for nickel.
A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
Definitions:
Freight-In
The cost associated with transporting goods into a warehouse or to the buyer, often included in the inventory cost.
Installation
The act of setting up or assembling machinery, equipment, software, or hardware to make it ready for operation.
Fully-Depreciated
A state where an asset has reached the end of its useful life for accounting purposes, with its book value equaling its salvage value or zero.
Leasehold Improvements
Enhancements made to a rental property by a lessee, typically to tailor the space to their specific business needs.
Q12: A microliter corresponds to:<br>A)10<sup>-2</sup> liters.<br>B)10<sup>-3</sup> liters.<br>C)10<sup>-6</sup> liters.<br>D)10<sup>-9</sup>
Q17: A piece of lead metal was added
Q53: Calculate the mass of excess reagent
Q60: Naphthalene combustion can be used to calibrate
Q83: Define Dalton's Law
Q99: An iron(II)ion has:<br>A)24 electrons and a charge
Q138: What is the formula for xenon hexafluoride?
Q142: The oxidation number of Fe in K<sub>3</sub>Fe(CN)<sub>6</sub>
Q151: What is the formula for xenon difluoride?
Q174: A 4.691 g sample of MgCl<sub>2</sub> is