The following data describes an initial and final state for an ideal gas.Given that the amount of gas does not change in the process, what is the final volume (mL)of the gas? 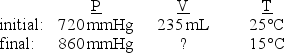
Definitions:
Proper Consideration
The requirement in contract law that something of value must be exchanged between the parties to form a valid contract.
Incorporeal Property
Refers to intangible assets owned by an individual or corporation, such as intellectual property, securities, and rights to financial accounts.
Nonprofit Corporation
A type of organization that operates without the purpose of making a profit, typically focusing on charitable, educational, or social missions.
Promoter
An individual or company responsible for organizing, financing, or sponsoring an event, project, or business venture, often prior to its creation.
Q8: The azide ion, N<sub>3</sub><sup>-</sup>, is very reactive
Q17: The Lewis dot symbol for the S<sup>
Q30: Balance the following and list the
Q84: Which of the following make an isoelectronic
Q86: What is the electron configuration of calcium?
Q87: If the radius of atom X is
Q89: Calculate the number of moles of cesium
Q100: Using the figure below, categorize electromagnetic radiation
Q114: Write the ground state electron configuration for
Q171: Write the balanced molecular and net ionic