Multiple Choice
At 25°C, the following heats of reaction are known: 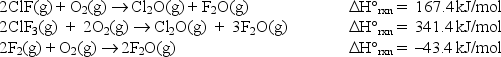
At the same temperature, use the above data to calculate the heat released (kJ) when 3.40 moles of ClF(g) reacts with excess F2: ClF(g) + F2(g) ClF3(g)
Definitions:
Related Questions
Q1: A possible set of quantum numbers to
Q2: What are the products of the
Q12: Calculate the wavelength associated with a <sup>20</sup>Ne<sup>+</sup>
Q25: In an effort to address concerns
Q86: In the following chemical reaction the
Q93: A molecule with 3 single bonds and
Q117: The second ionization energy of Mg is
Q120: A block of dry ice (solid CO<sub>2</sub>,
Q132: Which of the atoms listed below has
Q174: A 4.691 g sample of MgCl<sub>2</sub> is