Multiple Choice
The bond enthalpy of the Br-Cl bond is equal to H° for the reaction
BrCl(g) Br(g) + Cl(g) .
Use the following data to find the bond enthalpy of the Br-Cl bond. 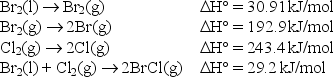
Definitions:
Related Questions
Q9: Consider the following reaction 2A +
Q11: Thorium metal is prepared by reacting
Q13: The radii of ions are always smaller
Q21: Concerning the electron configuration of P, 1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>4</sup>,
Q23: Thermal energy is<br>A)the energy stored within the
Q49: At body temperature 2,404 joules of energy
Q65: Calculate the wavelength of a neutron that
Q78: A 0.8715 g sample of sorbic acid,
Q80: Find <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q95: In the Lewis structure of the iodate