Given the following H° values,
H2(g)+ 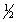 O2(g) H2O(l), H°f = -285.8 kJ/mol
O2(g) H2O(l), H°f = -285.8 kJ/mol
H2O2(l), H2(g)+ O2(g), H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+ 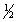 O2(g),
O2(g),
Definitions:
Information Management
The collection, storage, organization, and dissemination of information to support decision-making, coordination, control, analysis, and visualization in an organization.
Political Skills
The ability to understand and influence others in a way that promotes personal, team, or organizational objectives.
Developing
The process of growth, expansion, or evolution, often involving passage through various stages.
Internal Locus
The belief that one has control over their life and outcomes based on their own efforts and actions.
Q4: What type of reaction is the
Q10: Which one of the following is not
Q19: The effective nuclear charge (Z<sub>eff</sub>)felt by the
Q21: The mole fraction of oxygen molecules in
Q23: Of the following substances, KCl, KBr, and
Q29: The specific heat of gold is 0.129
Q45: Write the ground-state electron configuration for Br<sup>-</sup>.
Q78: If 2Mg(s)+ O<sub>2</sub>(g) <span class="ql-formula" data-value="\rarr"><span
Q103: For silicon atoms, which ionization energy will
Q129: The total number of valence electrons in