Calculate the standard enthalpy of formation of liquid methanol, CH3OH(l) , using the following information: 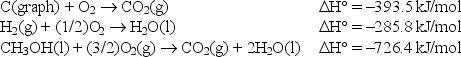
Definitions:
Repressed Unconscious
A concept in psychoanalytic theory referring to feelings, thoughts, and desires unconsciously held out of awareness, due to being deemed unacceptable or threatening.
Conditioning
A learning process in which an individual's behavior becomes dependent on or is triggered by a specific environmental stimulus.
Phobic Behaviors
Actions or reactions that are excessively fearful of specific objects, situations, or activities.
Reinforcement
In behavior psychology, it refers to any stimulus which strengthens or increases the probability of a specific response or behavior.
Q12: Ozone (O<sub>3</sub>)in the atmosphere can be
Q46: The following data describes an initial and
Q51: Which one of the following is
Q74: A 0.271 g sample of an unknown
Q78: Which of the following compounds is a
Q83: Identify the major ions present in an
Q92: Write the ground-state electron configuration for K<sup>+</sup>.
Q93: During a titration the following data were
Q105: The electron affinity of fluorine is essentially
Q133: Write a Lewis structure for the phosphate