At 25°C, the following heats of reaction are known: 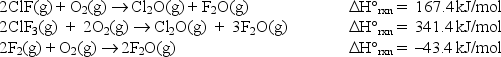
At the same temperature, use the above data to calculate the heat released (kJ) when 3.40 moles of ClF(g) reacts with excess F2: ClF(g) + F2(g) ClF3(g)
Definitions:
Bluing
A process used to protect steel against rust by forming a thin protective layer of magnetite (Fe3O4) on its surface.
Carbon Dioxide
A colorless, odorless gas produced by burning carbon and organic compounds and by respiration. It is naturally present in air and is absorbed by plants in photosynthesis.
Soda Pop
A sweetened, carbonated beverage, often flavored and containing caffeine, colours, and preservatives.
Mixture
A combination of two or more substances that are not chemically bonded and can be separated by physical means.
Q28: Identify the element being oxidized in
Q63: Which one of the following sets of
Q71: Phosgene, a chemical warfare agent used in
Q75: Calculate the volume occupied by 25.2 g
Q84: Given 2Al(s)+ (3/2)O<sub>2</sub>(g) <span class="ql-formula" data-value="\rarr"><span
Q94: Which of these gas molecules have the
Q105: Determine the oxidation number of each of
Q127: An FM radio station broadcasts at a
Q135: What is the energy in joules of
Q154: A 0.9182 g sample of CaBr<sub>2</sub> is