The bond enthalpy of the Br-Cl bond is equal to H° for the reaction
BrCl(g) Br(g) + Cl(g) .
Use the following data to find the bond enthalpy of the Br-Cl bond. 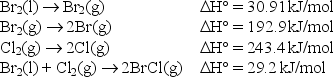
Definitions:
Government
The organization or system through which a community or nation is controlled and regulated.
Subsidized
Financial support provided by a government or organization to reduce the price of a product or service or to make it more available.
Central Utah Project
A United States federal water project undertaken by the Bureau of Reclamation to provide irrigation, municipal, and industrial water to parts of central Utah.
Traded
The action of buying, selling, or exchanging goods and services among parties.
Q1: What mass of Li<sub>3</sub>PO<sub>4</sub> is needed to
Q14: What is the molar mass of Freon-11
Q15: Arrange the following bonds in order of
Q91: Arrange the elements Ba, Br, and Ga
Q103: Give the number of lone pairs around
Q108: Identify the oxidizing agent in the
Q117: Today is a beautiful day for a
Q127: In general the effective nuclear charge felt
Q130: Write the ground-state electron configuration for O<sup>2-</sup>.
Q137: Calculate the mass of FeS formed