Given the following H° values,
H2(g)+ 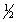 O2(g) H2O(l), H°f = -285.8 kJ/mol
O2(g) H2O(l), H°f = -285.8 kJ/mol
H2O2(l), H2(g)+ O2(g), H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+ 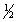 O2(g),
O2(g),
Definitions:
Yield Curve
A curve representing the yields of bonds with the same creditworthiness but varying expiration dates, measured at a specific moment.
Term Structure
The relationship between the interest rates or yields of bonds and their respective maturities.
Federal Government Bonds
Securities issued by a national government's treasury, often considered low-risk investments.
Risk Premium
The additional return expected by an investor for taking on a higher level of risk.
Q46: The elements in Group 2A are known
Q52: A convenient way to produce very
Q64: Which of the elements listed below would
Q73: A 45 mL sample of nitrogen gas
Q79: The reaction below can be classified
Q91: Which one of the following is
Q133: What mass of C<sub>12</sub>H<sub>22</sub>O<sub>11</sub> (sucrose)is needed to
Q134: Which element has the following ground-state electron
Q138: Which of the following elements has the
Q142: A molecule with 4 single bonds and